Oxygen dissolution and surface oxide reconstructions on Nb(100)
R. D. Veit, N. A. Kautz, R. G. Farber, and S. J. Sibener
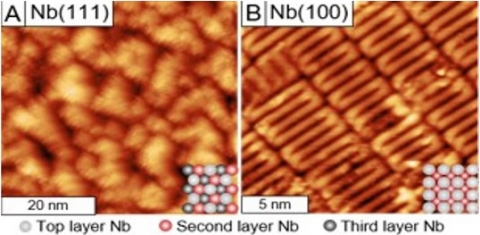
Niobium (Nb) superconducting radio frequency (SRF) cavity surfaces are terminated by a native bulk oxide layer whose composition and structure directly influences SRF cavity performance. This work demonstrated that the surface crystallographic orientation of Nb determines the structure of this oxide layer; Nb(100) formed well-ordered (3x1)-O ladder structures while NbO on Nb(111) had no discernible long-range order. The energy barrier for oxygen dissolution from NbO to the bulk was determined to be 0.6 eV, and structural evolution of the (3x1)-O surface as a function of oxygen dissolution was investigated using scanning tunneling microscopy (STM). It was shown that oxygen dissolution occurred preferentially at the midpoint of the (3x1)-O ladder structure, which may indicate a preferred surface site for oxygen dissolution.
Relation to CBB goals:
This work may have implications for SRF cavity fabrication procedures, resulting in higher cavity quality factors.
Potential applications:
SRF cavity fabrication procedures, such as nitrogen doping and Nb3Sn thin film growth, may be optimized with this understanding of how increased surface temperature influences the chemical and structural composition of Nb surfaces.
DOI:
ttps://www.sciencedirect.com/science/article/pii/S0039602819303589